The share of plastics used in the medical sector accounts for only around 2 percent of the total volume of plastics produced worldwide. Despite this comparatively low proportion of overall consumption, plastics have become firmly established as essential materials in medicine and medical technology. They are lightweight, X-ray and MRI compatible, physiologically safe, sterilizable, cost-effective, stable, and impact-resistant. In addition, they can be manufactured with high precision, in large quantities, and with consistently high quality in a wide range of colours and complex geometries.
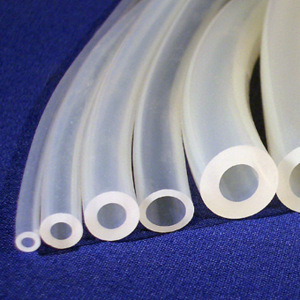
Suitable plastics are processed into items such as spoons and spatulas, sample containers and specimen jars, disposable syringes, and Luer lock connectors and are used in medical technology. Soft tubing, such as silicone tubing or tubing made of thermoplastic elastomers, is also used as tubing assemblies, whether for infusions or mechanical ventilation.
Requirements for Medical Plastics
Plastics used in medicine and medical technology must be processed into medical devices in their purest form, often under cleanroom conditions, and subsequently sterilized.
Medical plastics are classified into three categories based on application and duration of use: medical devices that come into contact only with the body surface, such as wound dressings or contact lenses; devices that come into contact with the internal body from the outside, such as disposable syringes, sutures, or catheters; and implants such as artificial joints, heart valves, or silicone implants. These categories are further subdivided according to the duration the medical device remains in the body. The shortest interval is less than 24 hours, the medium-term duration ranges from 24 hours to 30 days, and long-term use applies when the medical device remains in the body for more than 30 days.
Key Regulations for Plastic Products in Medicine
Plastic manufacturers must comply with the USP Class VI or ISO 10993 standards to ensure the biocompatibility of their products. USP stands for United States Pharmacopeia, a non-profit organisation that establishes standards for the quality and safety of medical devices, pharmaceuticals, and food products. USP Class VI is the most stringent classification and includes tests for acute systemic toxicity, intracutaneous reactivity, and implantation.
The ISO 10993 standard, published in Germany as DIN EN ISO 10993, imposes even stricter approval requirements. In addition to biological testing, it requires physico-chemical examinations and analyses of raw materials and extractables, and defines limit values for leachable substances.
The VDI Guideline 2017, issued by the Association of German Engineers (VDI), also serves as an important reference for medical device manufacturers. It defines the term “medical grade plastics” and outlines the properties and requirements for plastics used in medicine and medical technology. Applying this guideline supports quality assurance, formulation consistency, biocompatibility, and supply reliability. The guideline covers the areas of development, logistics, procurement, and purchasing alike.[1]
Which Plastics Are Used in Medicine?
Commodity Plastics: PE, PP, and PVC
The polyolefins polyethylene (PE) and polypropylene (PP) are considered physiologically safe and biocompatible and do not contain plasticizers. Plastic tubing made of PE and PP is transparent, flexible, and exhibits good chemical resistance.
Polypropylene is used for packaging solid and liquid pharmaceutical products, disposable syringes, laboratory containers, infusion sets, membranes for oxygenators, heart valve prostheses, and sutures. Polypropylene meshes are used in hernia surgery.
Polyvinyl chloride (PVC) is characterised by high transparency, a smooth surface, good kink resistance, favourable physiological properties, and low production costs. Soft PVC is used to manufacture bags, gloves, and soft tubing, for example for infusion solutions, blood, and urine.
However, plasticised PVC is controversial because it contains plasticizers such as diethylhexyl phthalate (DEHP), which are not chemically bound and can migrate from the material. DEHP is considered harmful to reproduction and has been banned in children’s toys and cosmetics. For medical technology, tri-(2-ethylhexyl) trimellitate (TEHTM, TOTM) has been developed as a substitute for DEHP. Rigid PVC is used as a sterilizable housing material for medical devices.
Silicone Rubber and PTFE
Polysiloxanes, organic silicon compounds, are temperature-resistant in a range from −70 °C to +250 °C, with some formulations even up to +300 °C. Silicone rubbers are characterised by very good biocompatibility and excellent elastic properties, are easy to sterilize, and do not contain plasticizers. In medical technology, they are used to manufacture pump tubing and ventilator tubing, respiratory masks, seals, hand splints, and foot insoles. In medicine, they are used as implants, pacemakers, heart valves, catheters, and drainage tubes. And in dentistry, they are used for making impressions of dental arches and jaws to produce precision models.
Polytetrafluoroethylene (PTFE), also known under the trade name Teflon® by DuPont, has an exceptionally low surface tension, which prevents the formation of thrombi. This medical plastic is used to manufacture vascular prostheses, ossicular prostheses, ureteral prostheses, and medical tubing. Due to its excellent sliding properties, it is also used as a coating material for catheters, cannulas, and injection needles.
Polystyrene and PMMA
Polystyrene (PS) is valued in medical technology as a transparent plastic for cell culture dishes and Petri dishes, containers for infectious or toxic materials, as well as packaging material. Expanded polystyrene (EPS), better known under the trade name Styropor®, serves as a transport container for refrigerated medications and vaccine sera.
In dentistry, polymethyl methacrylate (PMMA), better known under the trade name Plexiglas® by German company Röhm GmbH, plays an important role in the manufacture of prostheses, impression trays, and crowns. In endoprosthetics, this medical plastic is used as bone cement. The 1950s saw the first contact lenses made from PMMA. Since this material is impermeable to oxygen, silicone polymers are now used for rigid contact lenses.
Biodegradable Plastics
Another group of plastics used in medicine are biodegradable plastics, also called bioresorbable, which are broken down in the body after a certain period of time. These medical plastics include polylactic acid (PLA), polyglycolic acid, and polydioxanone. They serve as base materials for manufacturing vascular stents, wound staples, implants such as plates and screws for tissue and bone fixation, as well as suture material. The use of these bioplastics eliminates the need for a second surgical procedure to remove the implants.
Which Sterilization Methods Are Used for Which Medical Devices?
Whether disposable or reusable products, medical plastic components must be sterilized. Common sterilization methods include steam, hot air, radiation, plasma, and gas sterilization.
Steam and Hot Air Sterilization – Autoclaving of Plastics
During steam sterilization, also known as autoclaving, the components to be sterilized are heated at a pressure of approximately 2 bar and temperatures between +121 °C and +134 °C. Steam sterilization is suitable for many medical plastics.
In hot air sterilization, the plastic components are heated to high temperatures for a defined period of time. Common intervals are 2 hours at +160 °C, 60 minutes at +170 °C, and 30 minutes at +180 °C. However, this method can only be applied to temperature-resistant high-performance plastics such as polyetheretherketone (PEEK), silicone rubber, polysulfones, or polyetherimides (PEI).
Radiation and Plasma Sterilization
Radiation sterilization uses beta or gamma radiation. The radiation leaves no residues but can alter the polymer structure. This method is suitable for polyethylene and polysiloxanes; however, other plastics such as polypropylene, polyvinyl chloride, or polyoxymethylene may yellow and become brittle. Radiation sterilization is used almost exclusively for disposable products.

Plasma sterilization is carried out at low pressure in a vacuum chamber at temperatures between +50 °C and +60 °C. Additionally, vaporized hydrogen peroxide (H2O2) can be introduced, which also has a sterilizing effect. Since this method is carried out at low temperatures, it is suitable for almost all polymers that are vacuum-compatible and do not outgas.
Sterilization Using Ethylene Oxide
In ethylene oxide sterilization, medical devices are exposed to gaseous ethylene oxide in a sealed chamber at temperatures between +45 °C and +55 °C. Excess ethylene oxide is removed during an aeration phase. Since ethylene oxide is toxic, the products must be tested for residues after sterilization. Due to the low process temperature, this method is suitable for many polymers.
Polymers have made their way into medicine primarily as disposable products since the 1960s. As lightweight, stable, hygienic, and cost-effective products, they replaced glass and metal. Over time, they became established in many different areas of medicine, such as orthoses, prostheses, implants, and capsules for drug delivery. However, plastic products are also in demand as reusable items that can be sterilized and reused multiple times.
Sources: [1]: https://www.vdi.de/richtlinien/details/vdi-2017-medical-grade-plastics-mgp-1
Image Sources: Feature image | © angellodeco – stock.adobe.com Autoclave | © romaset – stock.adobe.com
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine