The term polyolefins refers to polymers produced from alkenes such as ethene, propene, butene, 2-methylpropene, and 4-methyl-1-pentene. Polyolefins are saturated hydrocarbons characterized by low density, good chemical resistance, low water absorption, and good electrical insulation. They are semi-crystalline thermoplastics that are easy to process and inexpensive to manufacture. Polyolefins account for the largest share of total plastic production and are therefore also referred to as standard or commodity plastics.
The group of polyolefins includes polyethylene (PE), polypropylene (PP), polybutylene (PB), polyisobutylene (PIB), and polymethylpentene (PMP). Numerous plastic articles are made from these cost-effective polymers, such as hoses, plastic sheets, packaging films and foils, housings for household and electronic devices, or plastic containers.
Polyethylene – the Most Common Commodity Plastic
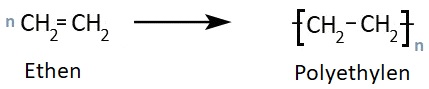
Polyethylene is produced by polymerizing ethene, also known as ethylene, and represents the polyolefin with the simplest repeating unit (-C2H4-)n.
This polymer was already discovered in 1898 by the German chemist Hans von Pechmann (1850 – 1902). However, the large-scale industrial production of this commodity plastic was only achieved in the 1950s with the discovery of the so-called Ziegler–Natta catalysts by Karl Ziegler (1898 – 1973) and the Italian chemist Giulio Natta (1903 – 1979). Both were awarded the Nobel Prize in Chemistry in 1963 for this achievement.

Polyethylene with only slightly branched polymer chains has the highest density, between 0.94 and 0.97 g/cm3, and the highest crystallinity, with a share between 60 and 80 %. It is designated PE-HD or HDPE, where HD stands for “high density”.
Polyethylene with highly branched polymer chains, on the other hand, has a lower density between 0.915 and 0.935 g/cm3 and a crystallinity between 40 and 50 %. It is labelled PE-LD or LDPE, meaning “low-density” polyethylene.
They show lower gas and water-vapor permeability than most plastics – only oxygen and carbon dioxide can diffuse through – and absorb very little water. With a melting point between +130 °C and +145 °C, both polyethylene types are easy to process but can be used permanently only within a limited temperature range up to +80 °C.
Compared to HD-polyethylene, LD-polyethylene has lower strength, stiffness, and hardness, but greater elongation and flexibility. It is mainly used as film material in the packaging industry and also for flexible plastic hoses. In contrast, piping, polyethylene hoses for pressure applications, and containers are generally made from HD-polyethylene.
In Germany, polyethylene accounts for 28% of all plastics consumed (approximately 32% in Europe). Further information about this polyolefin can be found in our magazine article Polyethylene – the most widely used plastic.
Polypropylene – the Second Most Common Commodity Plastic
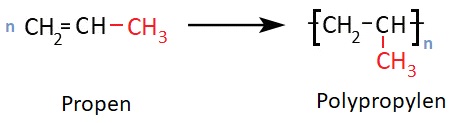
The starting material for synthesizing polypropylene (PP) is propene. The repeating unit of this polyolefin differs from that of polyethylene by an additional methyl side group.
Polypropylene was first synthesized in 1951 by the U.S. chemists J. Paul Hogan (1919 – 2012) and Robert Banks (1921 – 1989). As in the case of PE, large-scale industrial production became possible only after the introduction of Ziegler–Natta catalysts.
Depending on the polymerization conditions, the methyl groups can be arranged differently, as shown in the following structural formulas. When the methyl groups are arranged uniformly on one side of the polymer chain, the material is called isotactic polypropylene.
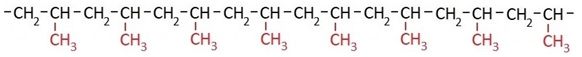
In syndiotactic polypropylene, the methyl groups are arranged alternately on both sides of the chain:
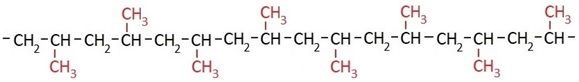
PP with randomly distributed methyl groups is called atactic polypropylene:
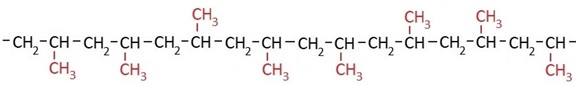
The methyl groups in polypropylene lead to higher chemical resistance and better mechanical properties compared with polyethylene. PP can be used at temperatures up to +121 °C. Because it is considered physiologically safe and easy to mold, it is primarily used for food packaging such as yogurt, curd, and margarine cups, for coating paperboard materials in milk and beverage cartons, and for closures on beverage bottles.
In the medical field, disposable products made of polypropylene are mainly used, such as spatulas and syringes. Furthermore, PP fiber materials serve as filter and screen fabrics, for example as particle filters in surgical masks.
In technical applications, plastic pipes and polypropylene hoses have become indispensable, as have metal-free small parts such as plastic hose connectors and fastening elements including washers, plastic screws, and hinges.
In automobile manufacturing, low-cost molded body parts made of polypropylene have proven their worth. To increase the strength of the material, glass or carbon fibers are often added to the colored plastic as fillers.
With a share of 17 %, polypropylene ranked second among the plastics consumed in Germany in 2017. Further information on polypropylene can be found in our magazine article Polypropylene: a versatile plastic (currently only available in German).
Polybutylene
Polybutene (PB), also called polybutylene, is obtained by polymerization of 1-butene. This polymer has an ethyl side group. As in the case of polypropylene, the side chains are predominantly isotactic, meaning they are arranged on one side of the polymer chain.
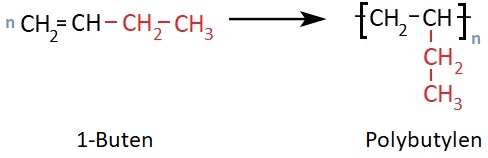
Polybutene was first synthesized in 1954 by the Italian chemist Giulio Natta and was introduced to the market in 1964 by Chemische Werke Hüls AG under the trade name Vestolen® BT.
PB is also physiologically safe, sound-absorbing, and characterized by high long-term strength. This means it can withstand constant pressure and temperature above the transition temperature for a long period before breaking.
Polybutene is mainly used as a material for plastic pipes in underfloor and surface heating systems, drinking water installations, pressure piping systems, and in district and local heating networks.
Polyisobutylene
The monomer 2-methylpropene, also known as isobutene, is the starting material for the synthesis of polyisobutylene (PIB). In 1931, the then Badische Anilin- und Sodafabrik (BASF SE) in Ludwigshafen-Oppau was granted a patent for the polymerization of isobutene to polyisobutylene. It has been produced since 1938 under the trade name Oppanol®.
Polyisobutylene has a structure similar to polypropylene but is bulkier due to the occupation of every second carbon atom by two methyl groups.
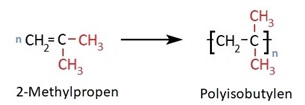
As a result, depending on the degree of polymerization, only viscous oils or rubber-like, plastic masses are obtained — but no solid polymers. Nevertheless, these too are valuable polyolefin products today.
Low-molecular-weight polymers such as PIB are mainly used as lubricating oils or lubricant additives and can be used permanently in a temperature range from −30 °C to +65 °C. Higher-molecular-weight polymers are employed as weather-resistant pastes and sealing compounds for large-area glazing in buildings and for durable cable coatings.
Polymethylpentene
Polymethylpentene (PMP) is produced by polymerizing 4-methyl-1-pentene. It differs from polypropylene in that the methyl group is replaced by an isobutyl group.
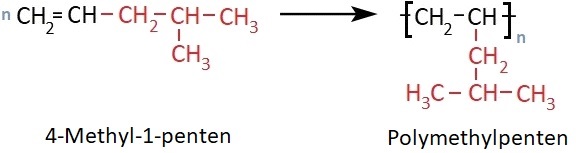
Polymethylpentene was first synthesized in 1956 by Giulio Natta. The British chemical company Imperial Chemical Industries (ICI) developed PMP to industrial maturity and introduced it to the market in 1967 under the trade name TPX®.
Polymethylpentene exhibits chemical resistance similar to that of polypropylene and can be used continuously at temperatures up to +120 °C. Due to the bulky isobutyl group in the molecule, however, its density is only 0.83 g/cm3, giving it the lowest density of all engineering plastics.
Its specific electrical resistance is roughly 100 times higher than that of all other polyolefins. PMP has very high permeability to moisture and gases — about ten times greater than polyethylene. The material also features outstanding non-stick properties, comparable to those of polytetrafluoroethylene (PTFE).
PMP is used as a material for laboratory utensils such as Petri dishes, funnels, measuring cylinders, storage containers, and beakers, as well as for films and containers in the food industry. PMP is also processed into crystal-clear barb connectors and reducer connectors for chemical engineering. More detailed information can be found in our magazine article PMP: Plastic with special properties (currently only available in German).
Recycling of Polyolefins
Compared with other plastics, polyolefins are inexpensive because they are derived from relatively simple olefins produced as by-products of steam cracking. They are considered environmentally compatible because they can be recycled. However, a plastic must have a purity level of at least 96 % to be recyclable.
Currently, about half of all polyolefin plastic waste is mechanically recycled, while the remainder is recovered for energy. The association “Polyolefin Circular Economy Platform” (PECP), a forum for all stakeholders in the polyolefin value chain, has set itself the goal of promoting the circular economy for polyolefins. Among its targets are the optimization of collection and sorting systems and achieving a recycling rate of around 75 % for polyolefin packaging by 2030.
A higher recycling rate can be achieved through improved separation of plastic waste. Researchers at Aarhus University, in cooperation with the Danish companies Vestforbrænding, Dansk Affaldsminimering Aps, and PLASTIX, have developed a “new camera technology capable of distinguishing between 12 different types of plastic”[1] — with higher accuracy than the near-infrared (NIR) spectroscopy currently used. This allows for greater purity of the separated plastic fractions.

A different approach is being pursued by trinamiX GmbH, a subsidiary of BASF AG and provider of mobile spectroscopy. It offers a “handheld kit” comprising a portable NIR spectrometer, a smartphone, data analysis in the trinamiX spectroscopy cloud, real-time access to results via a mobile app, and documentation in the customer portal[2]. This enables the sorting of plastic waste even in regions without dedicated sorting technology. Another important contribution to the polyolefin circular economy is the industry trend toward in-house recycling, where production waste is reprocessed in company-owned regranulation plants.
Text sources: [1]: https://www.chemie.de/news/1174212/durchbruch-bei-der-trennung-von-kunststoffabfaellen.html [2]: https://www.basf.com/global/de/media/news-releases/2022/11/p-22-400.html Image sources: Header image | © danielskyphoto – stock.adobe.com Giulio Natta | Unknown (Mondadori Publishers), Public domain, via Wikimedia Commons Plastic waste | © arnaudmartinez – stock.adobe.com
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine