Honey, water, ketchup and a starch solution represent four liquids with four very different flow behaviours. Honey flows slowly and thickly, but it can be made thinner by gentle heating. Water, on the other hand, seems to flow at a constant speed, while ketchup initially resists and does not flow until it suddenly becomes thin from one moment to the next. Finally, a starch solution is thin at rest but thickens when it is stirred continuously. The differing behaviour of these four liquids is described by their varying viscosities. This must not be confused with viscoelasticity, which describes the behaviour of solids and occurs frequently in plastics.


Short Definition of Both Behaviours
Viscosity
Viscosity describes the thickness or internal friction of fluids, more precisely the resistance of a liquid to a shear force. More accurately, this is referred to as shear viscosity to distinguish it from dynamic viscosity and kinematic viscosity.
Viscoelasticity
Viscoelasticity occurs in fluids and solids. A polymer melt, for example, can be viscoelastic. Viscoelastic material shows a mixed behaviour, partly viscous and partly elastic, combining characteristics of liquids and solids. Viscoelasticity depends on time, temperature and the frequency acting on a solid.
Derivation of Viscosity
The description and mathematical derivation of viscosity in liquids is often illustrated using the following model: a liquid is located between two plates separated by a defined gap, and the liquid is mentally divided into layers. One of the plates is moved parallel to the other stationary plate at a defined speed.
The top liquid layer close to the moving plate travels at nearly the same speed, decreasing continuously toward the stationary plate.
A force F is required for the movement of the liquid layers, which is ideally proportional to the area of the plate and the velocity and inversely proportional to the distance between the plates. The proportionality constant that relates these variables is called the dynamic viscosity. It is measured in the unit poise (P).
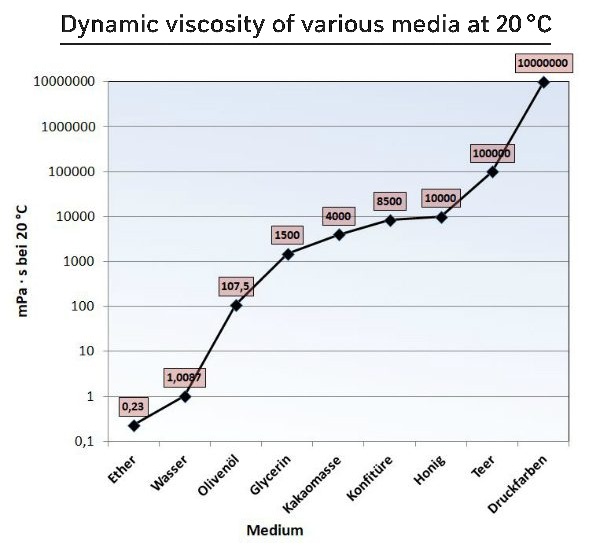
For practical applications, the unit centipoise (cP) is commonly used. For example, at +20 °C the viscosity of water is 1 cP, ethanol is 1.2 cP and honey is around 104 cP. Closely related to dynamic viscosity is kinematic viscosity, which is defined as the ratio of dynamic viscosity to the density of the substance and measured in stokes (St).

In practice, dynamic viscosity plays a major role in the transport of liquids through piping systems, for example in the long-distance transport of crude oil through oil pipelines.
Newtonian and Non Newtonian Fluids
As early as 1687, Newton studied the internal friction of liquids and described it as follows:
“The resistance arising from the lack of slip within a liquid is, assuming all other conditions remain equal, proportional to the velocity at which the liquid particles are separated from one another.”
All fluids that obey these laws are referred to as Newtonian fluids. Non Newtonian fluids, by contrast, do not show a linear relation between viscosity and applied speed but may change their behaviour due to shear force or time.
Types of Non Newtonian Fluids
Non Newtonian fluids can be divided into three groups: shear thinning fluids, shear thickening fluids and so called Bingham media.
Polymer solutions often exhibit the effect known as shear thinning, meaning that their viscosity decreases under higher shear forces. The individual polymer chains can more easily move past each other as shear increases, causing the solution to become thinner. These are known as shear thinning fluids.

In shear thickening fluids, viscosity increases with shear force. This is the case for starch solutions that become thicker when stirred with stirring devices.
A Bingham medium behaves like an elastic body below its yield point but becomes thinner over time once the yield point is exceeded. After the external force stops, it slowly returns to its original viscosity. A common example is ketchup, which must be shaken vigorously to start flowing and then gradually thickens again.


These behaviours must be considered when processing plastics at high temperatures, since they often behave like non Newtonian solutions.
Influence of Temperature
In addition to time and shear force, temperature has a major influence on the viscosity of fluids and gases. Viscosity is temperature dependent. It decreases in liquids as temperature rises, which must be considered when using lubricating oils.
The viscosity index is used in this context, describing the kinematic viscosity as a function of temperature.
Oils should have a high viscosity index to minimise temperature dependent variations. Even in gases, internal friction plays a role although it is weak due to the low particle density compared to liquids. It is the reason why the viscosity of gases increases measurably with temperature and pressure, both of which raise particle density.

How Exactly Does Viscoelasticity Behave?
Viscoelastic material changes its behaviour over time when subjected to external stress. Under constant load, the plastic deforms gradually, a phenomenon known as creep. The less common term is retardation.

In such cases, the plastic behaves like a liquid. At the same time, plastics are also elastic to a certain degree, meaning they return to their original form but not fully. The elastic component is responsible for spontaneous and reversible deformation, while the viscous component causes a time dependent, unlimited and irreversible deformation. The extent of viscoelastic behaviour differs between materials, as does the way in which the two components interact.
Viscoelasticity is also temperature dependent, as the processes occur more rapidly at higher temperatures.
This is especially important when assessing ageing processes. Viscoelasticity plays a major role when selecting gaskets or O rings. Since the material gradually loses its shape, plastics must be chosen whose viscoelasticity meets the specific requirements.


In summary, viscosity and viscoelasticity are two key properties that significantly influence the behaviour and application range of solutions, fluids and materials.
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine
