Sintering is a manufacturing process that has been known and widely used for a very long time. The production of ceramics – such as firing kaolin or shaping clay into pottery – are sintering processes discovered empirically and continuously refined since their origin. Today, this process is applied not only to ceramics, especially technical ceramics, but also to the production of sintered materials made from metals and plastics. Sintering enables the production of semi-finished products and specialised components, such as sintered plates, discs and tubes for filtering aggressive gases and liquids. These are used not only in chemical laboratories but also in the chemical, biotechnological and pharmaceutical industries. In addition, shaped components made of sintered materials – especially sintered metals – are widely used in mechanical and automotive engineering.
The Principle of Sintering
The sintering process can be roughly described as “fusing together” powdered or granulated raw materials at elevated temperatures. Upon closer examination, however, the process is more complex and takes place in several phases.
By selecting the raw material, its grain size, and defining the process parameters, the pore size can be precisely controlled.
Phase 1: Shaping
First, the powdered or granulated raw materials are shaped or pressed into so-called “green bodies”, which already take on the form of the desired sintered products. It is important to achieve a homogeneous packing density at this stage and ensure that the powder particles adhere firmly to one another. If this is not the case, an organic binder must be added. In principle, three basic methods are available for producing a green body: dry or wet pressing of the raw materials under high pressure, slip casting using water-based slurries, and what is known as plastic forming.


The Pressing Process
Pressing the raw materials at pressures of one tonne per square centimetre and above can be done either dry or wet. In dry pressing, the moisture content of the material is below 7%. In wet pressing, it exceeds 12%, requiring the green body to be thoroughly dried before sintering
The appropriate pressing method depends, among other factors, on the geometry of the semi-finished part.
Wet pressing is recommended for more complex shapes, while dry pressing is generally sufficient for simpler parts. In uniaxial pressing, a punch compresses the powdered material to about one third of its original volume. Because the pressure is applied from only one side, the resulting density is not uniform throughout.

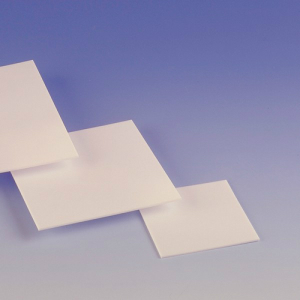
Uniaxial pressing is therefore mainly used for the production of flat, plate-shaped sintered parts made from polyethylene (HDPE), polytetrafluoroethylene (PTFE), bronze or Cr-Ni steel. For more uniform density, double-sided pressing (also known as co-axial pressing) is used, applying pressure from both the top and bottom punches. This method can be used, for example, to produce axially oriented magnets.

During pressing, magnetic powders are already aligned by a magnetic field. This anisotropic orientation remains during the subsequent sintering. If pressure is applied evenly from all directions, the process is called “isostatic pressing”.
Materials produced this way show high, uniform density and isotropic properties – meaning they behave the same in all directions.
Casting Processes
Besides pressing, several casting techniques are also used to create green bodies, especially when complex geometries are required. The production of ceramics, earthenware or porcelain are examples of slip casting, where a slurry of the raw material in water is poured into plaster moulds. The mould absorbs the water, leaving a solid green body that can be further processed. Another method is cold casting, where a binder such as epoxy resin is added to the mass.


The ceramic injection moulding process is an example of plastic forming. A thermoplastic polymer is added to the ceramic powder as a binder and injection-moulded into the desired shape. The green body is then sintered, during which the plastic burns away. A similar process, metal injection moulding, uses fine metal powders (e.g., chrome, nickel or iron powders or alloys like brass and stainless steel) instead of ceramic powders.
This method enables the efficient, precise production of complex parts.
Phase 2: Densification
After shaping, whether by pressing, casting or plastic forming, sintering takes place. Green bodies must be pre-dried, especially those made by wet pressing. Then the actual sintering process begins. Different materials require different sintering temperatures, usually just below their melting point. For technical ceramics, temperatures can reach up to 1600 °C, while metals and metal alloys generally sinter at lower temperatures. Sintering typically occurs in three phases under a protective gas atmosphere or vacuum.


Particle Rearrangement
At the beginning of sintering, diffusion and rearrangement lead to the formation of initial contact points between the particles. So-called “necks” form as the particles of the starting material bond together, thereby reducing their total surface area. This process is energetically favourable and thus occurs spontaneously. As a result, the density of the material gradually increases.
As a rule of thumb, an increase of 10% in density can be expected.
Material Densification
In the second phase, these necks grow as the particles enlarge and fuse more completely, resulting in significant densification – up to 30%. Pores or channel systems form, allowing trapped gases to escape.

Reduced Porosity
The third phase leads to a more closed and dense pore structure. Particle growth continues, but excessive “grain growth” may occur, potentially impairing the material’s properties. At this stage, further densification occurs only slightly, so the sintering process must be carefully controlled and stopped in time.
Subsequent Steps – What Follows the Actual Sintering Process?
After sintering, the parts cool down. Further processing may follow, particularly if the components must meet specific technical tolerances. This may involve pressing the parts again at high pressure and temperature.
Compared to conventional forming processes, post-processing is often unnecessary, as sintered components can already meet precise specifications.
Applications of Sintering
Sintering is an efficient and material-saving method for manufacturing a wide range of products – from ceramic components for the electronics industry to precision parts made of sintered metal used in the automotive sector, such as brake pads.

Sintering is also used for producing magnets and dental ceramics. Sintered plates, discs and tubes made of plastics like HDPE, LDPE or PTFE are used in chemical laboratories and equipment construction for clear filtration of process solutions and as air filters in cleanroom technology.
Laser Sintering – A Process for Plastics
This specialised sintering technique is used for certain plastics. The granulate is applied layer by layer and sintered immediately. This enables the creation of fine three-dimensional structures.


Thanks to the wide variety of available materials, sintering is a universally applicable forming process. It is also highly material-efficient, with no waste – all of the input material is retained in the final product.
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine
