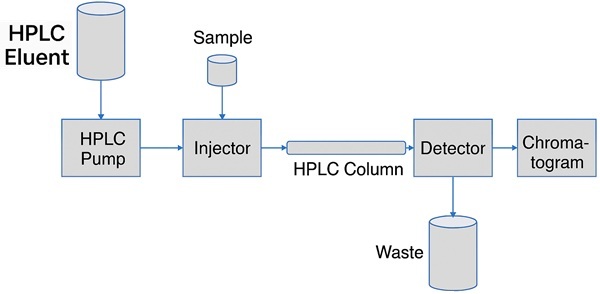
HPLC stands for High Performance Liquid Chromatography. This technique is used for both preparative and analytical separations. In biochemical research, HPLC is especially useful for preparing and analyzing substances with high molecular weight, such as proteins.
What Is Liquid Chromatography?
Chromatography is a separation technique in which a mixture is divided between two phases: a stationary phase and a mobile phase. In liquid chromatography, the stationary phase is a solid, while the mobile phase is a liquid, commonly referred to as the eluent.
As the mobile phase flows past the stationary phase, substances within the mixture interact differently with the surface of the stationary material. These varying interactions lead to different flow velocities and ultimately to separation.
Various materials can serve as the stationary phase: for instance, special filter papers in paper chromatography, or silica gel and aluminum oxide in column and thin-layer chromatography. Mobile phases range from non-polar solvents like hexane to polar solvents such as water.
How Does HPLC Work?
HPLC is essentially an advanced form of column chromatography. In this method, a high-pressure pump (typically 50–400 bar) pushes the mobile phase through a column filled with the stationary phase, ensuring smooth and pulse-free flow.
Once a sample is introduced via an injector at the start of the column, separation occurs inside the column. A downstream detector records a signal proportional to the amount of each component eluted and presents this data in a graphical format known as a chromatogram. Ideally, each peak in the chromatogram corresponds to a distinct component of the mixture.

Commonly used detectors include UV-absorbance, refractive index, and fluorescence detectors. Recycling uncontaminated eluents helps reduce solvent consumption significantly.
Compared to traditional gravity-driven column chromatography, HPLC works with smaller sample volumes, much faster elution rates, and considerably higher resolution. Heating the column via a column oven can alter interactions between mobile and stationary phases, thus influencing separation performance.

Ultra-high-performance liquid chromatography (UHPLC) takes things a step further by using even smaller particles and shorter, narrower columns—further increasing resolution.
The following section provides a brief overview of the key components in a high-performance liquid chromatography system:
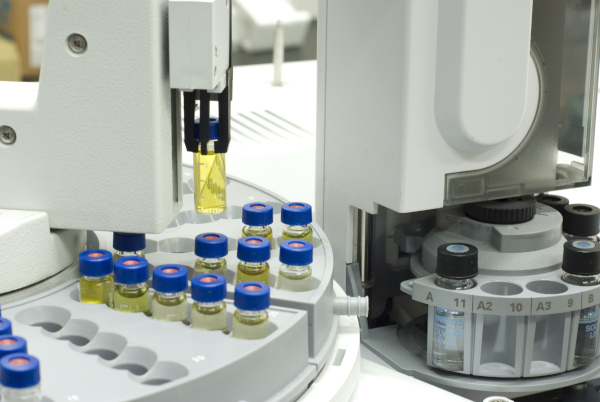
- Sample Injection
Samples are typically injected using fixed-volume sample loops. A six-port valve switches the flow path so that the eluent pushes the sample from the loop onto the column.
At low to medium pressure, manual injection using syringes is possible. In automated settings, autosamplers minimize manual workload. The injected volume depends on the column’s diameter and typically ranges from 1 µL to 2 mL.
- Chromatography Column
Since pressures up to 400 bar are applied, columns are made from thick-walled glass, often sheathed in pressure-resistant stainless steel for safety. However, stainless steel can interact with bioactive substances, making it unsuitable for many biochemical applications. For this reason, bioinert materials such as titanium are sometimes used. Internal diameters usually measure only a few millimeters.


- Stationary Phase
HPLC columns are densely packed with solid, porous, and pressure-stable materials—typically functionalized silica gels. In contrast to low-pressure chromatography, the particle size in HPLC is significantly smaller, usually < 10 μm, which improves resolution.
Separation may be based on adsorption, ion exchange, or size exclusion, allowing for a wide range of applications.

The polarity of both phases strongly influences retention time—the time a substance takes to travel through the column. A substance with weak interactions with a non-polar stationary phase has a shorter retention time.
Although normal-phase HPLC can be difficult to manage (e.g. due to water contamination in solvents), reverse-phase HPLC is far more commonly used in practice.

- Mobile Phase
Polarity charts and elutropic series can help select appropriate eluents. Mixtures of solvents are often used to fine-tune polarity and elution strength. Electronic mixers allow for dynamic gradient programming of solvent composition.
Applications of High-Performance Liquid Chromatography
- Analytical Use
Identification is typically done by comparing a sample’s retention time with that of a reference substance. Mixing the sample with the reference can confirm identity: a single, larger peak suggests a match; two peaks indicate non-identity. Repeating the process with a different mobile phase helps rule out accidental peak overlaps.
Even greater certainty is achieved by switching the stationary phase (e.g., from NP- to RP-HPLC). However, full confirmation requires qualitative identification of the eluted substance. This can be accomplished using specialized detectors such as mass spectrometry or diode-array systems that record entire UV/Vis spectra.
- Preparative Use
HPLC is also used for purification—just like classical column chromatography. This is common in pharmaceutical or biological sample preparation. Larger internal diameters are used to accommodate the greater volumes typical of preparative applications.
Relevance in Biochemistry
HPLC’s versatility stems from its wide array of separation modes and detection methods. In biochemical laboratories, it’s indispensable for analyzing complex mixtures of biopolymers—such as nucleic acids, amino acids, peptides, or enzymes. With proper scaling, HPLC can also purify target compounds from complex matrices. It is therefore a standard tool in modern biochemical research.
HPLC is also used in medical diagnostics, for example, in determining vitamin D levels in blood samples.
Other typical applications include polymer analysis, detection of active ingredients and contaminants, purity testing, and quantitative analysis of substances in biological samples. HPLC is also commonly used in drinking water testing. HPLC is routinely employed in environmental monitoring and water quality testing across Europe.
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine
