Fully demineralised water, also known as deionised, demi-water, demineralised, distilled, or ultrapure water — all look just like regular tap or spring water but contain very few or no foreign substances at all. In contrast, tap or spring sources always contain a significant amount of dissolved salts. These include cations like magnesium (Mg2+), calcium (Ca2+), sodium (Na+), or potassium (K+), and anions like chloride (Cl–), bicarbonate (HCO3–), or sulfate (SO42–). These ions contribute to the electrical conductivity and are therefore an indicator of purity.
The conductivity indicates the degree of purity. The lower the conductivity, the higher the purity. Conductivity is usually given in microsiemens per centimeter (µS/cm). According to drinking water regulations in Germany, the limit is 2,500 µS/cm at +20 °C. By comparison, seawater conductivity varies by region between 45,000 µS/cm and 60,000 µS/cm.
Distillation – The Oldest Method of Purification
Distilled water, as the name suggests, is produced through distillation, where the water is heated to its boiling point and the steam is collected and condensed. The distillate is practically free of salts and largely free of organic substances as well. The conductivity of this water is below 20 µS/cm and can be further reduced through multiple distillations using quartz apparatus. However, distillation is a very energy-intensive process.
Methods for Producing Deionised Water
Deionised water can be produced by ion exchange or by reverse osmosis.
Fully Deionised Water Through Ion Exchange
In ion exchange, the water to be purified flows through cation and anion exchange resins or through mixed bed exchangers that contain both types of resins. Mixed bed exchangers, also called mixed bed filters, are mainly used in industrial applications.

In this process, cations are exchanged for hydrogen ions (H+) and anions for hydroxide ions (OH–). Once the exchange resins are saturated and can no longer absorb ions, they must be regenerated or replaced. For regeneration, diluted hydrochloric acid (HCl) or diluted sulfuric acid (H2SO4) is used for cation exchangers, and diluted sodium hydroxide (NaOH) or potassium hydroxide (KOH) for anion exchangers. The conductivity of deionised water obtained this way is below 5 µS/cm.
Demineralization by ion exchange does not remove organic substances, viruses, or bacteria. To remove these, an additional filtration step is necessary.
Deionised water is also called demi-water, or demineralised, water. If it is also sterilized, it is marketed as distilled-equivalent.
Deionised Water Through Reverse Osmosis
Reverse osmosis is a filtration method in which the water to be purified is forced through a semipermeable membrane. Semipermeable means that the membrane allows water molecules to pass but blocks dissolved salts, particles, organic impurities, viruses, and bacteria.
Osmosis refers to the one-sided diffusion of a substance through a semipermeable membrane without external pressure. Imagine two aqueous solutions with different salt concentrations separated by a semipermeable membrane. Water will diffuse from the lower concentration solution to the higher concentration solution until the concentrations equalize. The force with which the solvent of the less concentrated solution is attracted to the more concentrated solution is called osmotic pressure.
If pressure greater than the osmotic pressure is applied to the more concentrated salt solution, the osmotic process is reversed. Since the membrane only allows water molecules to pass, only these migrate from the more concentrated solution to the less concentrated one. As a result, the salt concentration in the more concentrated solution continues to increase, while the less concentrated solution becomes even more diluted. This process is called reverse osmosis.
In desalination by reverse osmosis, such as the demineralization of drinking water, the process runs not against a low-concentration salt solution but against pure water. Since the salt concentration on the side of the non-demineralised water keeps increasing, it must be flushed at regular intervals. This prevents excessive salt buildup and simultaneously removes retained organic impurities and particles into the wastewater. The conductivity of deionised water produced by reverse osmosis ranges between 5 and 20 µS/cm.
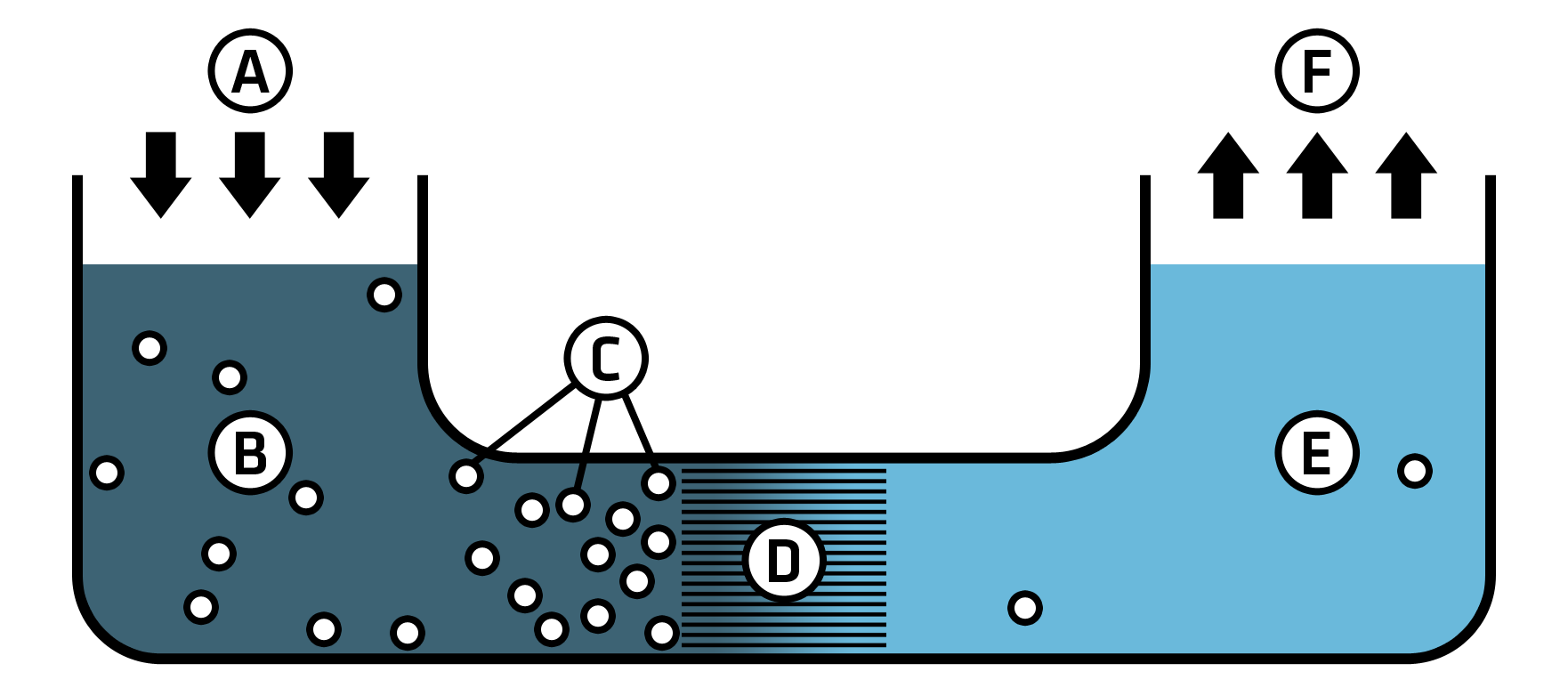
While ion exchange requires chemicals to regenerate the exchange resins and produces wastewater containing chemicals, reverse osmosis uses significant amounts of water for flushing, which is then discharged as wastewater.
The advantage of ion exchange compared to reverse osmosis is its efficiency: one liter of drinking water yields one liter of deionised water, whereas with reverse osmosis, less than 750 ml of deionised water is obtained from one liter of drinking water.
Ultrapure Water – The Highest Level of Purity
Producing ultrapure water is complex and requires combining multiple purification steps. First, larger particles and suspended solids are removed by filtration, followed by demineralization through reverse osmosis or ion exchange.
Ultrafiltration then removes microparticles, organic substances, and colloids. UV irradiation at 254 nm eliminates germs and bacteria, sterilizing the water. This results in ultrapure water with a conductivity below 0.1 µS/cm that is sterile and germ-free.
Due to the removal of all metal ions, ultrapure water is very aggressive toward many metallic alloys. Pipes made of copper or brass are attacked by this medium, dissolving ions from the metals.
Therefore, physiologically safe and chemically inert rubber tubing or plastic tubing — such as drinking water hoses — is often used for ultrapure systems. Appropriate fittings like tube & hose connectors, taps and valves are also made from suitable polymers. Alternatively, various stainless steels can be used for ultrapure lines.
Which Water for Which Application?
The required purity always depends on its intended use.
For most applications, fully demineralised water is entirely sufficient. In chemical and biochemical laboratories, as well as in many technical areas, deionised water is the standard rinsing and solvent medium. For example, in car washes, deionised water is typically used in the final rinse to prevent lime spots on the car’s paint.
Many households, especially those in mountainous regions where the supply is particularly rich in minerals, have their own “softening or decalcification system.” In these systems, tap water flows through an ion exchange resin bed that replaces calcium and magnesium ions and other multivalent cations with sodium ions. This turns “hard” tap water into “soft” water for domestic use, minimizing scale buildup in household appliances like heaters, boilers, and kettles. Washing machines and dishwashers are often equipped with their own cation exchangers to prevent gradual scaling of the appliances, especially their heating elements.
For some applications, however, ultrapure water is indispensable. Especially in the semiconductor industry, where structures between a few nanometers and a few micrometers are produced, using ultrapure water is essential for manufacturing computer chips and integrated circuits.
In pharmaceutical production, for manufacturing injection solutions, and for diluting solutions in sensitive research fields like biochemistry, microbiology, and genetic engineering, extremely high standards are required for water quality. According to the European Pharmacopoeia, the electrical conductivity at +20 °C must not exceed 1.1 µS/cm. In addition, nitrate content must not be higher than 0.2 mg/l, the total organic carbon (TOC) must not exceed 0.5 mg/l, and the level of bacterial endotoxins, which indicates the presence of bacteria, must be below 25 ng/l.
Image sources: Header image | © Sakan – stock.adobe.com Purification system for demineralised boiler water | © Z22, CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons – stock.adobe.com Reverse osmosis schematic: By Colby Fisher - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=25213532
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine




