Among the fundamental tasks in a chemical laboratory are the preparation of solutions and the dilution of existing ones. A solution is defined as a homogeneous mixture of at least two substances. The substance present in the largest proportion is referred to as the solvent, while the substance present in a smaller proportion is known as the solute.
Polar substances such as salts, sugars, or urea preferentially dissolve in polar solvents, whereas nonpolar substances such as oils or waxes dissolve in nonpolar solvents.
Key Quantities in the Chemical Laboratory
The proportion of the dissolved substance in a solution is specified by its concentration. A distinction is made between mass concentration and amount-of-substance concentration. The unit of mass concentration is g/L, while that of amount-of-substance concentration is mol/L. It is also common to specify mass percent, which indicates the mass of the dissolved substance in grams per total mass of the solution.
What Is the “Solubility” of a Substance?
Another important term in the chemical laboratory is solubility. According to the CRC Handbook of Chemistry and Physics, solubility describes the mass of a compound that can dissolve in 100 g of water at a specific temperature. Because solubility depends on temperature, the temperature to which the stated value applies is always specified. If a solution contains the maximum amount of dissolved substance at a given temperature, it is referred to as saturated. Any additional substance added beyond this point remains undissolved and settles at the bottom.
What Is Meant by a Volumetric Solution, Standard Solution, or Calibration Solution?
A solution with a precisely defined concentration is referred to as a volumetric solution, standard solution, or calibration solution. The term “volumetric solution” is used in volumetric analysis, also known as volumetry or titrimetry. In such solutions, the concentration remains constant, they react rapidly with the analyte, and the reaction proceeds to completion. Calibration or standard solutions are used in instrumental analysis to verify or adjust measuring instruments such as pH meters or conductivity meters.
Preparation of Volumetric Solutions
Volumetric solutions can be prepared from primary standard substances. Primary standards are highly pure substances that can be weighed accurately and are indefinitely stable. They must not change due to exposure to oxygen, carbon dioxide, or moisture from the air. A volumetric solution prepared from a primary standard must remain stable over an extended period. Alternatively, concentrates for preparation or ready-to-use solutions are available from laboratory suppliers.
For volume measurement of liquids, calibrated laboratory containers such as pipettes, volumetric flasks, and graduated beakers and cylinders are used in the chemical laboratory. To prepare a volumetric solution, the previously calculated mass of the substance is weighed on an analytical balance and quantitatively transferred into a volumetric flask. The flask is filled to about three quarters with the solvent and gently swirled until the substance has completely dissolved. It is then carefully filled to the calibration mark, sealed, and mixed again.
In analytical laboratories, it is often not possible to prepare a solution with an exactly defined concentration. The deviation of the actual concentration from the theoretical value is expressed by a correction factor known as the titer. The titer is the ratio of the experimentally determined actual concentration to the theoretical concentration. The volume of standard solution consumed during a titration is multiplied by the titer. For standard solutions not prepared from primary standards, the titer must be determined experimentally using a suitable primary standard substance.
Because the preparation of standard solutions from primary standards is time-consuming, laboratory suppliers offer glass or plastic ampoules under trade names such as Fixanal® or Titrisol®. These ampoules contain concentrates for preparing many commonly used standards. The ampoule is placed on a volumetric flask, pierced with a glass rod, and its contents are quantitatively transferred into the flask. The flask is then filled to the calibration mark with distilled water. According to the manufacturer, determination of the titer is not required; a titer deviation of ±0.2% is specified.
Use of Reagent Solutions
An important application of reagent solutions is qualitative analysis, where they are used for the rapid identification of substances. One example is the Fehling test. It is used to detect reducing agents such as aldehydes, hydrazine, or hydroxylamine, and for the determination of sugars in urine. For this test, Fehling’s solution I, a diluted copper sulfate solution, Fehling’s solution II, an alkaline potassium sodium tartrate tetrahydrate solution, and the test solution are combined. The initially blue mixture is then heated. In the presence of reducing agents, red-brown copper(I) oxide, Cu2O, precipitates after heating.
The Mixing Cross: Preparing Diluted Solutions
The concentration of concentrated and diluted acids or bases is often expressed in mass percent. For example, concentrated sulfuric acid contains 96%, fuming hydrochloric acid 37%, and concentrated nitric acid 65% by mass. Diluted preparations can be obtained from these concentrates.
To calculate the volumes of solutions with different concentrations required for mixing or dilution, the mixing cross is used in the chemical laboratory. The mass fraction of the more highly concentrated solution 1 is placed at the upper left, and that of the less concentrated solution 2 at the lower left. The mass percent of the target solution is placed in the center. The proportion of starting solution 1 is obtained from the difference between the mass percent of the target solution and that of starting solution 2. Accordingly, the proportion of starting solution 2 is calculated from the difference between the mass percent of starting solution 1 and that of the target solution.
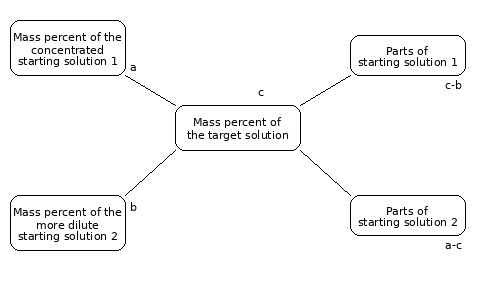
If 10% sulfuric acid is to be prepared by diluting concentrated sulfuric acid with distilled water, the concentration of starting solution 1 is 96%, that of starting solution 2 is 0%, and the target concentration is 10%. From the mixing cross, the mass fractions are 10 parts concentrated sulfuric acid and 86 parts water. Using 10 g of concentrated sulfuric acid and 86 g of distilled water, 96 g of 10% sulfuric acid can be prepared.
A 10% sulfuric acid solution is chemically less aggressive and compatible with a wide range of laboratory components, such as hoses and hose connectors made of various plastics. In contrast, 96% sulfuric acid is extremely corrosive and can only be used with chemically resistant materials such as glass and fluorinated plastics like PTFE (polytetrafluoroethylene).
When several solutions of different concentrations are prepared from a stock solution, this is referred to as a dilution series. These are used in spectroscopic methods such as atomic absorption spectroscopy (AAS) or optical emission spectroscopy (OES) to generate calibration curves. In a subsequent measurement, a sample with an unknown concentration is analyzed, and its concentration is determined from the calibration curve.
Because volume measurement is performed visually, it is prone to error. Laboratory suppliers therefore offer dispensing devices that weigh the required mass of the substance to be dissolved, transfer it into the target vessel, and dispense solvent until the selected concentration is reached.
Solutions Outside the Chemical Laboratory
Solutions play an important role not only in chemical laboratories. Especially during the COVID-19 pandemic, 70% isopropanol/water solutions were widely used as disinfectants in hospitals, medical practices, and everyday settings.
In addition to cleaning agents used in electronics, medical technology, and households, they are also used as nail polish removers in the cosmetics industry. Ethanol is used as a solvent in the production of liquid medications because active ingredients often do not dissolve in water. In everyday life, we encounter solutions in the form of cleaning products such as drain cleaners, toilet cleaners, or descaling agents, as well as in alcoholic beverages.
Image sources: Title Image | © H_Ko – stock.adobe.com
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine