Carbon is located in the second period and fourth main group of the periodic table of the elements. This position gives it something of a special role, since it can form four atomic bonds. This enables a very large number of different bonding possibilities. In addition, carbon atoms can form bonds with each other, which greatly increases the number of possible combinations. The following article examines two of these possibilities, also called modifications: the carbon modifications diamond and graphite.
Minerals Of A Special Kind
Diamond and graphite are modifications of the element carbon, meaning elemental substances that consist only of carbon atoms. Only the arrangement of the atoms in the two modifications differs. Graphite is a mineral that occurs relatively frequently on Earth. A piece of graphite consists only of carbon atoms that are connected to one another via covalent bonds. In such a bond, the strongest type of all bonding types, the two bonded atoms share the valence or outer electrons. Diamond is also a mineral, but far rarer. Just like graphite, the exclusively occurring atoms of this element in diamond are covalently bonded to each other.

Diamond – The Eternal Carbon?
In the James Bond classic “Diamonds Are Forever” (1971), diamond jewelry swallowed by people withstands the heat of a crematorium. But the famous French scholar Antoine L. Lavoisier (1743 – 1794) had already disproved this much earlier by burning a diamond in front of the astonished eyes of the Parisian spectators on the street. Using two large burning lenses, like a magnifying glass, he focused the sun’s rays onto a diamond placed in a glass flask and it burned. In addition, the experimental chemist succeeded in detecting carbon dioxide (CO2) as the combustion product in the apparatus he built himself. This was considered proof that diamonds consist only of carbon.
Diamond is the hardest of all naturally occurring substances. Diamonds are colorless and do not conduct electricity. They are found, among other places, in sometimes dangerous locations such as volcanic vents – but why?

The conditions that prevail there, such as high pressures and temperatures, promote the formation of this modification of carbon. Only a small portion of these raw diamonds is suitable for the production of jewelry. Most are used in the form of drill bits and cutting tools as industrial diamonds. However, because high temperatures often occur during machining, processing, for example, steel with diamond tools is hardly possible. The extreme conditions cause a conversion from the modification diamond into graphite, which can lead to diffusion of the carbon atoms and thus contamination of the material.
However, diamonds can also be artificially produced by compressing graphite under high pressure and temperature.
The Structure Of Diamonds
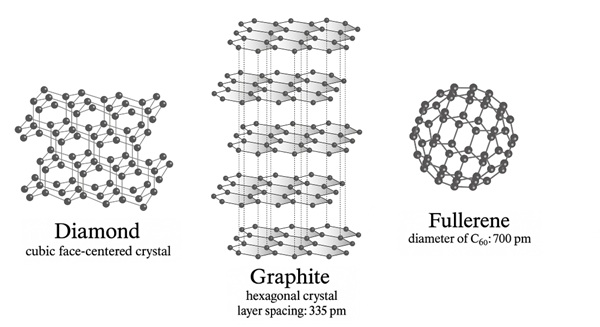
Carbon atoms in diamond form a three dimensional lattice, also called the diamond lattice. With its four valence electrons, that is electrons in the outermost shell of an atom, a carbon atom forms four electron pair bonds with four other carbon atoms. The resulting tetrahedral structure leads to the occurrence of wavy six membered rings. Four of the atoms in such a six membered ring form a planar rectangle, one of the two remaining carbon atoms lies above and the other below this plane. These electron pair bonds, also called covalent bonds, are very strong, which is why a great amount of energy must be applied to break them. This explains, among other things, the exceptional hardness of the mineral diamond.
Graphite – The Most Common Form Of Carbon
The pencil tip consists, contrary to what the name might suggest, not of lead but of graphite. Graphite is a black, very soft substance that shines in crystalline form. When gliding over paper, small flakes split off and leave behind a black, slightly silvery shiny trace. It is due to this property that graphite is used as pencil lead as well as a lubricant. The name of graphite itself, derived from the Greek word “graphein,” meaning “to write,” also stems from this characteristic. Graphite is also used to manufacture electrodes and as a sliding contact in the form of carbon brushes in electric motors. Unlike the modification diamond, graphite is able to conduct electricity due to its structure.

The Structure Of Graphite
The different arrangement of the atoms in the two modifications of this element results in the partly very different properties of diamond and graphite. This graphite lattice consists of flat layers lying on top of each other, composed of flat six membered rings of carbon atoms. Three valence electrons of each carbon atom participate in bonds with three further atoms. The fourth electron is similar to the electronic structure of metals, in which the electrons are distributed across the entire structure in what is technically referred to as the electron gas, and it is able to move freely across the entire layer. This particular spatial structure means that graphite conducts electricity parallel to these layers but acts as an insulator horizontally to them.
The distance between the different layers is more than twice as large as the distance between the atoms within the same layer. The special softness and the possibility of use as a writing and lubricating material are based on the low attractive forces between the layers, which are much weaker compared to the forces between the atoms within a layer.
Carbon And Other Carbon Based Materials
The structure of graphite and the associated properties of this modification of carbon are very similar to the properties of what is colloquially referred to as “carbon”. However, this often refers to carbon fibers, which are produced by controlled carbonisation of synthetic fibers and in which carbon atoms are arranged in microcrystals similar to graphite.


Carbon fibers are five times as strong in tension as steel and far more resistant to corrosion. They are used in many areas in which the special properties of this structure of carbon are required. They are often combined with plastics to form carbon fiber reinforced plastics (CFRP). These are significantly lighter than aluminium and are used where low weight is important, such as in automotive and aircraft construction. In high performance sports, carbon fiber reinforced plastics are used in the form of tennis rackets, pole vault rods and bicycle frames.


Further possibilities for the use of modifications of carbon exist in the processing of diamond like carbon by applying a very thin layer by means of CVD (chemical vapour deposition) onto components in order to protect them from wear. Introducing other non metals and metalloids such as nitrogen or boron into the diamond structure makes diamonds conductive and thus enables their use in semiconductors and superconductors. The combination of increased temperature resistance and comparatively free mobility of the electrons in the structure is beneficial. An acoustic and less optical use of diamonds is found in the higher priced pickup cartridges of long playing records (LP), where they provide the transmission of sound to the speakers.
Image sources: Header image | © Björn Wylezich – stock.adobe.com Pure graphite | © Ra'ike de.wikipedia.org Natural diamonds | © Mario Sarto de.wikipedia.org Carbon modifications | © natros – stock.adobe.com Pencil tip made of graphite | © Helfmann de.wikipedia.org
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine
