What do car tires and chewing gum have in common? Both contain the same type of rubber: butyl rubber. The development of butyl rubber began in 1825 with the discovery of a flammable gas during the distillation of petroleum by the English scientist Michael Faraday (1791–1867). This gas was a mixture of isomeric butenes: n-butene, cis-2-butene, trans-2-butene, and isobutene (2-methylpropene). The first synthetic rubber based on the polymerization of isobutene was developed by the German Badische Anilin- und Sodafabrik (BASF) and introduced to the market in 1931.
This rubber-like polymer, polyisobutylene, was named Oppanol after its production site in Oppau (Germany) and is abbreviated as PIB. Oppanol remains part of BASF’s core business today and is used as a sealing material, adhesive, and coating material.
This synthetic rubber exhibited better technical properties than polyisobutylene and was launched in 1937 under the name “butyl rubber.” Today, butyl rubber is mainly produced by ExxonMobil and Lanxess, which was spun off as an independent company from Bayer AG in 2004.


In the 1950s and 1960s, halogenated butyl rubbers were developed by dissolving butyl rubber and treating it with chlorine or bromine. These so-called “halobutyls,” abbreviated CIIR or BIIR, can be vulcanized more rapidly and with smaller amounts of vulcanizing agents. In addition, they allow improved co-vulcanization with other elastomeric polymers and increase the crosslinking rate.
Structure of Butyl Rubber
Butyl rubber is a polymer belonging to the group of synthetic rubbers. The correct technical term for butyl rubber is isobutylene–isoprene rubber, abbreviated IIR. It is a copolymer of 2-methyl-1-propene (isobutylene) and 2-methyl-buta-1,3-diene (isoprene). The following figure schematically illustrates the structure of the two monomers and the resulting polymer.
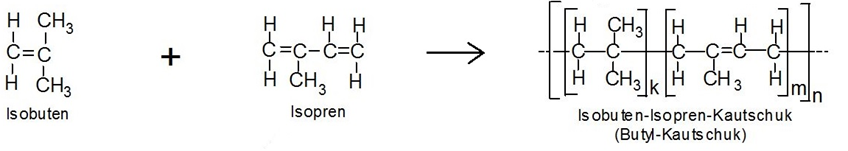
The indices “k” and “m” represent the molar fractions of the two starting components in the repeating unit of the macromolecule, which can repeat “n” times. In practice, the proportion of isobutylene is much higher than that of isoprene, as resistance to aging and weathering decreases with increasing isoprene content.
The isoprene content determines the number of double bonds in the polymer backbone. This assigns IIR to the R group of synthetic rubbers, which are characterized by double bonds in the main polymer chain. These double bonds can be used for halogenation to halobutyl rubber (chlorobutyl or bromobutyl) or for vulcanization.
Production of Butyl Rubber
Butyl rubber is produced by exothermic chain polymerization of isobutylene with small amounts of isoprene in solution. Dichloromethane (CH₂Cl₂) is most commonly used as the solvent. The resulting polymer is insoluble in the solvent and precipitates out.

The length of the polymer chain depends strongly on the polymerization temperature. Since chain length has a significant influence on the technical quality of the final product and increases as temperature decreases, butyl rubbers are polymerized at very low temperatures ranging from −40 °C to −100 °C. Aluminum(III) chloride (AlCl3) or boron trifluoride (BF3) are used as catalysts. As a raw product, butyl rubber is available uncolored in the form of off-white to yellowish bales, but it can also be supplied colored, most commonly black or white.
Properties and Processing
Butyl rubber is an elastic polymer and has the lowest gas permeability of all rubbers.
It can be used in a temperature range from −30 °C to +100 °C, and for short periods even up to +140 °C. Isobutylene–isoprene rubber (IIR) provides good electrical insulation, with a specific volume resistivity greater than 1012 Ω·cm. Depending on the base material, Shore A hardness ranges between 35° and 85°, which is typical for elastomeric polymers. The elongation at break of IIR is up to 700%, placing it at the upper end of the range for elastomers.
Isobutylene–isoprene rubber is resistant to acids and alkalis but not resistant to oils and greases. It exhibits good damping properties against vibration and impact energy. Butyl rubber with a low isoprene content is weather- and ozone-resistant. As the isoprene content increases, weather and ozone resistance decrease, while heat resistance increases.
Application Examples of Butyl Rubbers
Butyl rubber is used in a wide range of applications. The primary customer is the tire industry. In this sector, the rubber is used in inner tubes as the inner rubber layer—also referred to as an “inliner” or “inner liner”—as well as in tubeless tires. It is also used for curing bladders in tire manufacturing.
Due to its damping properties, IIR is also used in mounts and elastic joints in the automotive industry to isolate vibrations between the chassis and the body.
In the construction industry, butyl rubber is used in the form of tapes, profiles, and adhesives. Butyl adhesives are used for sealing and insulation. Joint and sealing tapes coated with butyl rubber are used to seal concrete joints against ground moisture and pressurized water. Bridge cables are protected against corrosion by wrapping them with butyl rubber tapes. Insulating glass units are sealed with butyl rubber profiles. Rubber mats and rubber sheets made of IIR are also used for sealing applications.


Because of its good electrical insulation properties, butyl rubber is used as cable sheathing, for repairing damaged cable or wire jackets, and in cable duct seals for sealing cable and pipe penetrations.
In the pharmaceutical industry, container stoppers as well as seals and O-rings are manufactured from butyl rubber. In the food industry, IIR is the base material used in the production of chewing gum.
And in sports and leisure applications, butyl rubber is found in balls and shoe soles.
Hoses made of butyl rubber are used as gas hoses in the chemical industry and in laboratories due to their low permeability to gases and water vapor. Gloves made of isobutylene–isoprene rubber are used as laboratory gloves because of their resistance to acids and alkalis. In protective suits and gas masks, IIR is used in sealing components. Containers are lined with IIR to protect against acids and alkalis.
Alubutyl, an aluminum foil coated with butyl rubber, is used in automotive engineering, loudspeakers, and surround sound systems for damping and sound insulation.

In 2013, Lanxess opened a butyl rubber plant in Singapore with an annual capacity of 100,000 metric tons of butyl and halobutyl rubber. Approximately three quarters of sales are generated by the tire industry, while around ten percent is attributed to the pharmaceutical industry. In some Asian countries, such as China, halobutyl rubber is legally required as a material for closures and seals in pharmaceutical products. In addition to Singapore, Lanxess also produces butyl rubber in Sarnia (Canada) and Zwijndrecht (Belgium), each with an annual capacity of 150,000 metric tons.
Image sources: Featured image | © tong2530 – stock.adobe.com Butyl Rubber gloves | By MatChem121 – Own work, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=14643835 Aluminum-coated butyl sheets | © mehmet – stock.adobe.com
 Reichelt Chemietechnik Magazine
Reichelt Chemietechnik Magazine
